Abstract
Background: Patients with relapsed or refractory (R/R) acute lymphoblastic leukemia (ALL) and lymphoblastic lymphoma have an unmet need for new treatment strategies. Navitoclax (Nav), a BCL-2/BCL-XL/BCL-W inhibitor, has shown promising effects in hematologic malignancies, however its usage is limited by prolonged thrombocytopenia mediated by inhibition of BCL-XL in platelets (J Clin Oncol. 2012;30:488). Venetoclax (Ven) is a highly selective BCL-2 inhibitor with similar anti-tumor efficacy. The addition of Nav to Ven has shown synergistic effects in preclinical models and might mitigate the dose-limiting thrombocytopenia associated with Nav monotherapy (Blood. 2016;128:1382). We report updated outcomes for ALL patients treated with Ven, Nav, and chemotherapy.
Methods: R/R ALL patients aged ≥4 years were enrolled in this phase 1, multicenter, open-label, dose escalation study (NCT03181126). Patients received the weight-adjusted equivalent of 200 mg Ven on day 1, and a 400 mg equivalent daily thereafter. Daily oral Nav was administered on day 3 onward with up to three dose levels for patients ≥45 kg (25, 50, 100 mg) and up to two dose levels for patients <45 kg (25, 50 mg). Patients could also receive chemotherapy from day 9 onward, consisting of peg-asparaginase (1250 IU/m2 intravenous [IV] on days 9 and 22), vincristine (1.5 mg/m2 IV on days 9, 15, 22, and 29), and dexamethasone (20 mg/m2/day orally on days 9-13 and 22-26) at the investigators' discretion. Disease assessment was made by flow cytometry on day 8 and day 36 and as clinically indicated. Minimal residual disease (MRD; < 10-4 cutoff for MRD-negativity) evaluation was performed at time of disease assessment, if clinically indicated.
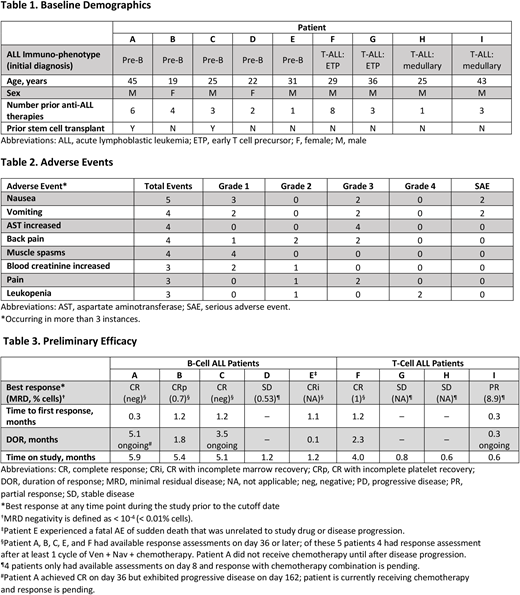
Results: Nine adult patients, ≥45 kg (5 with B-cell ALL and 4 with T-cell ALL) have been enrolled as of the data cutoff of June 1, 2018. All patients treated thus far have received 400 mg QD Ven and 25 mg QD Nav (Dose Level 1). Chemotherapy was started on day 9 for 7 patients; patient A began chemotherapy on day 169 and patient H began on day 10. Baseline characteristics of the first 9 patients are shown in Table 1. The most common adverse events (AEs) of any grade are shown in Table 2. Serious AEs included nausea, vomiting, febrile neutropenia (n=2 each), abdominal pain upper, pseudomonal sepsis, somnolence, septic shock, acute pancreatitis, and pulmonary embolism (n=1 each). Of the serious AEs, only febrile neutropenia was considered possibly related to Ven + Nav. AEs led to dose interruptions in 4 patients. No dose-limiting toxicities (DLTs) have occurred. As of the data cutoff, 2 patients have died. Patient B died due to disease progression on day 148 and Patient E died due to an event of sudden death on day 38 not related to study drug (event was associated with not wearing a LifeVest personal defibrillator). Preliminary efficacy for these 9 patients is shown in Table 3. All 9 patients were evaluated at day 8 after Ven + Nav therapy alone. One patient had a complete response with incomplete marrow recovery (CRi) and 1 patient had a partial response (PR); the remaining patients had stable disease (SD). Five patients had assessments at day 36 or later, including 4 patients who had completed 1 cycle of chemotherapy combined with Ven + Nav, and 1 who had received Ven + Nav only; 3 patients achieved CR, and 1 patient each achieved CRi and CRp as their best response. Patient A achieved CRi after Ven + Nav therapy without chemotherapy on day 8, had a duration of response of 5.1 months before developing progressive disease and is now also receiving chemotherapy. Patient C achieved CR on day 36, stopped all therapy on day 38, and maintains ongoing response at 3.5 months. Overall, in this heavily pretreated group, 5 patients achieved a complete response (CR/CRi/CRp). Two patients with CR had no detectable MRD. The remaining 4 patients have not reached the day 36 assessment as of the data cutoff; of these patients, 1 patient had PR and 3 patients had SD.
Conclusions: Ven + Nav in combination with chemotherapy is well tolerated, without any unexpected side effects and no DLTs observed to date. Preliminary data suggest that Ven + Nav with chemotherapy is efficacious in some patients with R/R ALL who have had multiple lines of therapy, including prior stem cell transplant. Enrollment, clinical follow-up, and correlative biology studies are ongoing.
Alexander:Abbvie: Other: travel expenses. Jabbour:Bristol-Myers Squibb: Consultancy, Research Funding; Abbvie: Research Funding; Takeda: Consultancy, Research Funding; Novartis: Research Funding; Pfizer: Consultancy, Research Funding. Khaw:Abbvie: Research Funding; Amgen: Other: travel expenses, Research Funding; Bristol Myers Squibb: Research Funding; Jazz Pharmaceuticals: Research Funding; Novartis: Other: travel expenses. Mullighan:Cancer Prevention and Research Institute of Texas: Consultancy; Abbvie: Research Funding; Amgen: Honoraria, Speakers Bureau; Loxo Oncology: Research Funding; Pfizer: Honoraria, Research Funding, Speakers Bureau. Leonard:Amgen: Research Funding. Schmidt:Abbvie: Employment, Equity Ownership. Tong:Abbvie: Employment, Equity Ownership. Zhou:Abbvie: Employment, Equity Ownership. Ross:AbbVie, Inc: Employment, Equity Ownership. Rosenwinkel:Abbvie: Employment, Equity Ownership. Jacobson:Abbvie: Employment, Equity Ownership. Kim:Abbvie: Employment, Equity Ownership. Stock:Jazz Pharmaceuticals: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal